Definition Of Quanta Energy Levels
Translational energy levels The translational energy levels of a molecule are usually taken to be those of a particle in a three-dimensional box. A fixed orbit implies a fixed energy and thus no falling of electron into the nucleus.
Planck S Constant Readings Planck S Constant Photoelectric Effect Wave Particle Duality Bohr Atom Accelerating Electron Produces Em Radiation Light Loses Energy And Spirals Into Nucleus I E Atom Should Not Work The Uv Catastrophe And The Dilemma Of
Spectral theory characterises what they.

Definition of quanta energy levels. From its use in physics meaning the sudden jump of an electron atom etc. The energy characteristic of a stationary state of a physical system especially a quantum mechanical system. 3 energy levels and quanta 1.
If the hydrogen atom could possess any energy then it could lose any amount of energy and emit a photon of any energy and frequency. When we describe the energy of a particle as quantized we mean that only certain values of energy are allowed. Both involve a relatively heavy nucleus with electrons moving around it although strictly speaking the Bohr model works only for one-electron atoms or ions.
With multiple electrons there is an additional source of splitting of the electron energy levels which is characterized in terms of another quantum number the total anglular momentum quantum number JThe source of the splitting is called the spin-orbit effectFor light atoms the spins and orbital angular momenta of individual electrons are found to interact with each other strongly enough. Cairogina2003 cairogina2003 10092017 Chemistry High School answered Explain the connection between energy quanta and energy levels. When we consider hydrogenic atoms with nuclear charges greater than one we must allow for the increased attraction between the nucleus and the electron and the resultant change in the energy.
Quantum energy level synonyms Quantum energy level pronunciation Quantum energy level translation English dictionary definition of Quantum energy level. The energy levels are by definition the eigenvalues of such operator in its domain of definition mathcalD_H. What is the value of Quanta.
Transition energies and energy levels in hydrogen The energy of a given atomic orbital is therefore proportional to the inverse square of the principal quantum number. 1973-2005 Edit Credential Planks energy equation E hf gives the fundamental quantum of energy by putting f1 in the Planks equation. Enxnynz h2 2m nx 2 lx 2 ny2 ly nz2 z 2 In general the separation of the translational energy levels is many orders of magnitude smaller than kT.
Values between those quantized values are not permitted. Energy Levels and Quanta1 2. Dadarao Dhone former Retiree Executive EngrEM.
Plancks idea that they can only possess certain properties such as energy and angular momentum in discrete amounts quanta. The energy characteristic of a stationary state of a physical system especially a quantum mechanical system. Energy LevelsPlanks and Einsteins quantum theory of light gives thekey to understanding the regular patterns in line spectraPhotons in these line spectra have certain energy valuesso electrons in those atoms can only certain energy valuesThe energy level diagramshows a very simple case itis for an atom in.
As the intensity of electromagnetic energy increases or decreases it steps up or down from one quantized level to another rather than follow a smooth and continuous curve. Quantum energy definition quantum energy meaning. Molecular energy levels and spectroscopy 1.
Quantized energy means that the electrons can possess only certain discrete energy values. That works out E h the Planks. The fundamental notion that a physical property can be quantized is referred to as the hypothesis of quantization.
Not in a continuous spectrum 2 The photoelectric effect showed that. In physics a quantum plural quanta is the minimum amount of any physical entity physical property involved in an interaction. And some said the Energy of a wave is the sum of its quantas energy.
The level near the to nucleus has lower energy as compared to the far one. Therefore the energy of the electron in a hydrogen atom must be restricted to certain energy levels. Energy level synonyms energy level pronunciation energy level translation English dictionary definition of energy level.
Now its really starting to sound like photons. From one energy level to another quantum mechanics n functioning as sing. Click here to get an answer to your question Explain the connection between energy quanta and energy levels.
Perhaps a particle can only have 1 Joule 4 Joules 9 Joules or 16 Joules of energy. Black body radiation could only be explained by assuming that the radiation came in quanta ie. The quantization of energy refers to the absorption or emission of energy in discreet packets or quanta.
The Hydrogen atom spectrum also tells us what these energy levels are. But this is not observed. The orbit is defined as an energy level at which the electrons remain and are allowed to transit from one level to another.
The main things that confuse me are the quantaphoton-wave relationit sounds like photons are parts of waves instead of having waves describing them which is what I thought in the beginning and what the wave function describes.
Planck S Constant Readings Planck S Constant Photoelectric Effect Wave Particle Duality Bohr Atom Accelerating Electron Produces Em Radiation Light Loses Energy And Spirals Into Nucleus I E Atom Should Not Work The Uv Catastrophe And The Dilemma Of
Planck S Constant Readings Planck S Constant Photoelectric Effect Wave Particle Duality Bohr Atom Accelerating Electron Produces Em Radiation Light Loses Energy And Spirals Into Nucleus I E Atom Should Not Work The Uv Catastrophe And The Dilemma Of

What Is A Quantum Of Energy Quora
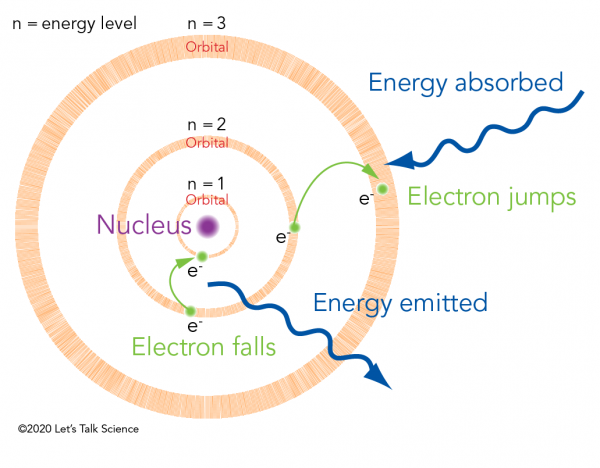
Introduction To Quantum Mechanics Let S Talk Science
Planck S Constant Readings Planck S Constant Photoelectric Effect Wave Particle Duality Bohr Atom Accelerating Electron Produces Em Radiation Light Loses Energy And Spirals Into Nucleus I E Atom Should Not Work The Uv Catastrophe And The Dilemma Of

Energy Quanta Development Of Quantum Physics Electrical4u
Planck S Constant Readings Planck S Constant Photoelectric Effect Wave Particle Duality Bohr Atom Accelerating Electron Produces Em Radiation Light Loses Energy And Spirals Into Nucleus I E Atom Should Not Work The Uv Catastrophe And The Dilemma Of

Energy Quanta Development Of Quantum Physics Electrical4u

Energy Quanta Development Of Quantum Physics Electrical4u

Energy Quanta Development Of Quantum Physics Electrical4u

Atomic Energy Levels Video Khan Academy

Quanta And Wave Particle Duality Quantum Theory And The Uncertainty Principle The Physics Of The Universe

Atom Orbits And Energy Levels Britannica

Quanta And Wave Particle Duality Quantum Theory And The Uncertainty Principle The Physics Of The Universe



Post a Comment for "Definition Of Quanta Energy Levels"